Pharmaceutical Regulatory Affairs Consulting
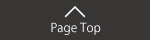
Pharmaceutical life cycle and task at each step
Each step in the pharmaceutical life cycle involves the following tasks:
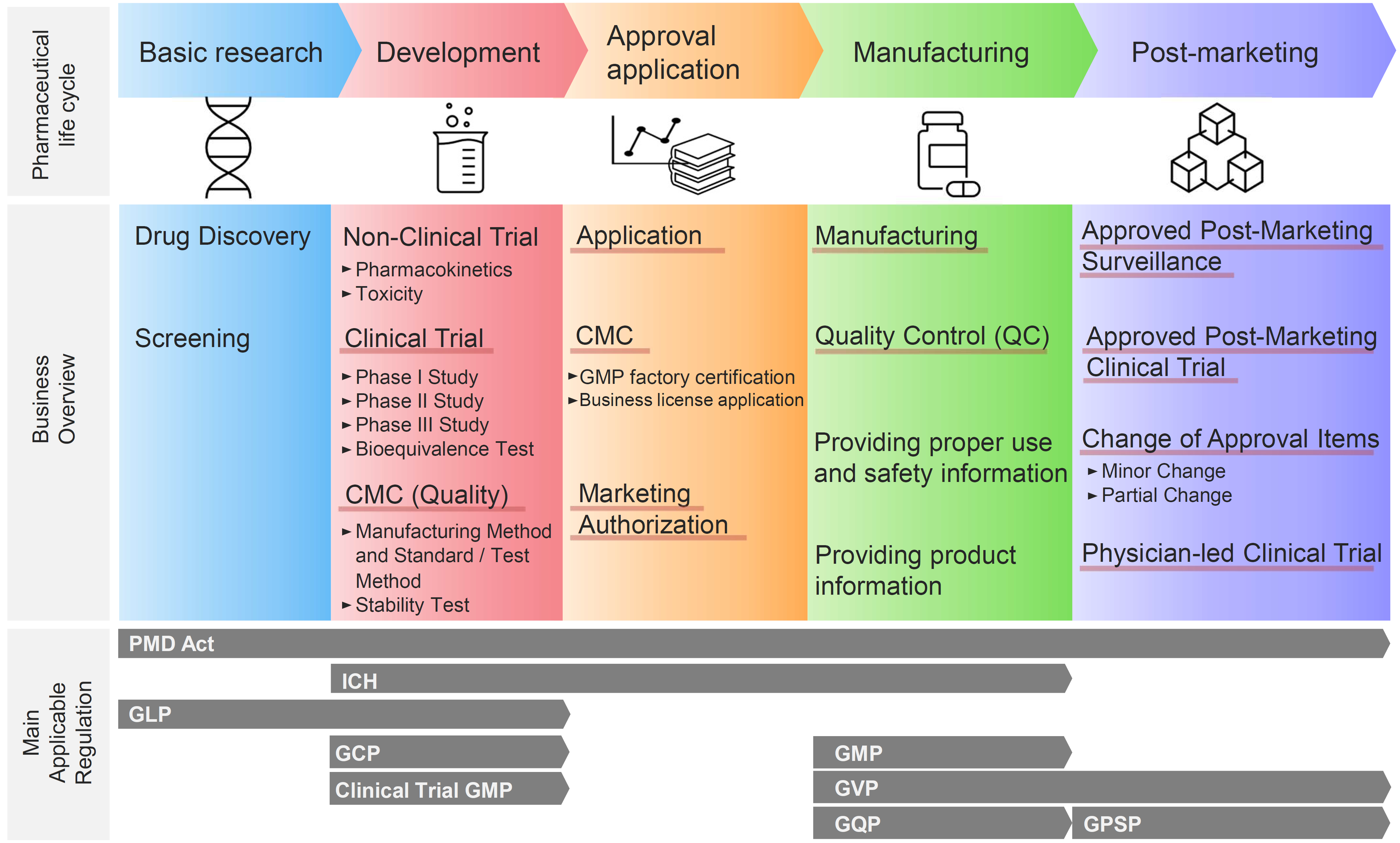
* The red underline indicates the scope of CM Plus's regulatory consulting service.
Pharmaceutical Regulatory Affairs Consulting from CM Plus
Our experts offer pharmaceutical regulatory affairs consulting services with abundant practical experience of the industry to meet our customers' needs.
-
■Regulatory Application Strategy
-
■Building Internal Systems such as GQP / GVP
-
■Various Consultations with the Pharmaceuticals and Medical Devices Agency (PMDA)
-
■Support for Manufacturing/Marketing Business License for Pharmaceutical Products etc./ Manufacturing Business License/ Application for Accreditation of Foreign Manufacturers (including Support for Inquiry Response)
-
■Business Support for Creating Approval Application Forms and Application Materials
-
■Business Support for Partial Change, minor change (including support for inquiry response)
Customer’s Benefits from CM Plus Pharmaceutical Regulatory Affairs Consulting
(1) Regulatory Affairs Consulting Services at all stages, from Development to Post-Marketing
CM Plus provides consulting services for regulatory affairs development, CMC regulatory affairs, and pharmaceutical regulatory affairs at all stages, from the development stage, approval application, manufacturing, to post-marketing. We can handle new drugs, generic drugs, long-listed drugs (after the re-examination period), and various types of drugs.
(2)Regulatory Affairs Consulting Services in Collaboration with GMP Consulting Services.
CM Plus provides two services. The first is "GMP consulting services, from the perspective of GMP manufacturing and QA quality assurance department" (on-site support). The second is "pharmaceutical regulatory affairs consulting services, from the perspective of regulatory interpretation and document procedures" (we provide consulting service of regulatory response to regulatory authorities, from application to inquiry response). Combining these two services enables us to provide collaborative GMP, QA, and regulatory consulting services that are more suitable for the site.
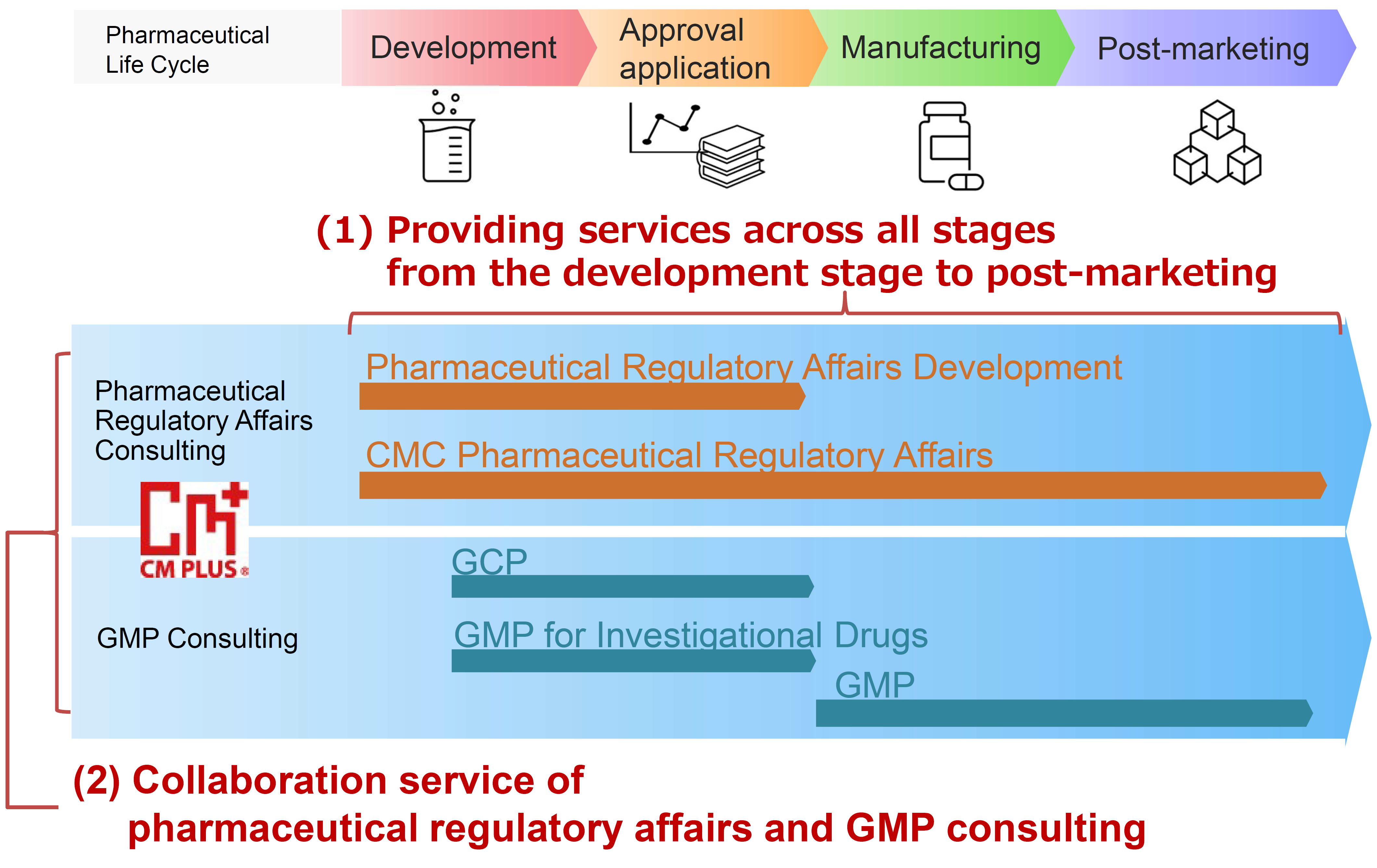
Designated Marketing Authorization Holder
and In-Country Clinical Caretaker
CM Plus has obtained the first-class and second-class marketing licenses for drug. We can be appointed as Designated Marketing Authorization Holder (D-MAH) for companies that have no company established in Japan. We also provide services any support for clinical trials in Japan that conform to Japan GCP Ministerial Ordinance as In-Country Clinical Caretaker (ICCC: Article 15 in GCP Ministerial Ordinance) (sponsor role in Japan)
Designated Marketing Authorization Holder (D-MAH) Service
This service enables foreign manufacturers to enter the Japanese market for pharmaceutical products voluntarily.
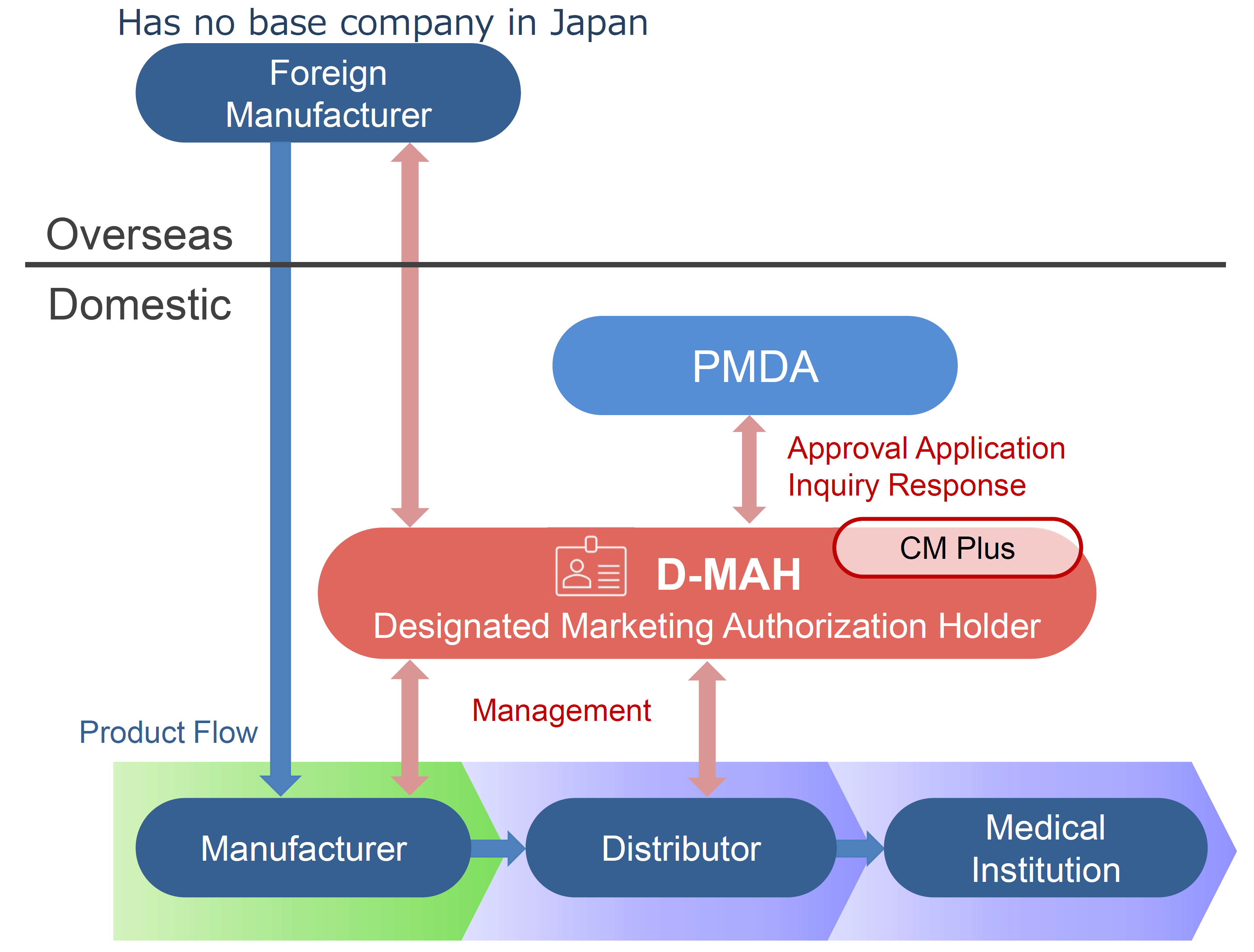
In the case that a foreign manufacturer does not have a base for business operations in Japan for the sales of pharmaceuticals and medical devices, it is necessary to appoint one manufacturing/marketing business license holder in Japan.
CM Plus has obtained the first-class and second-class marketing licenses for pharmaceuticals. We will provide services as a Designated Marketing Authorization Holder (D-MAH) for foreign manufacturers who have entered the Japanese market and consider importing and selling.
In-Country Clinical Caretaker (ICCC) Service
This service enables clinical trials to be conducted in Japan on behalf of overseas pharmaceutical companies (pharmaceutical companies, drug discovery ventures, etc.) without a legal entity.
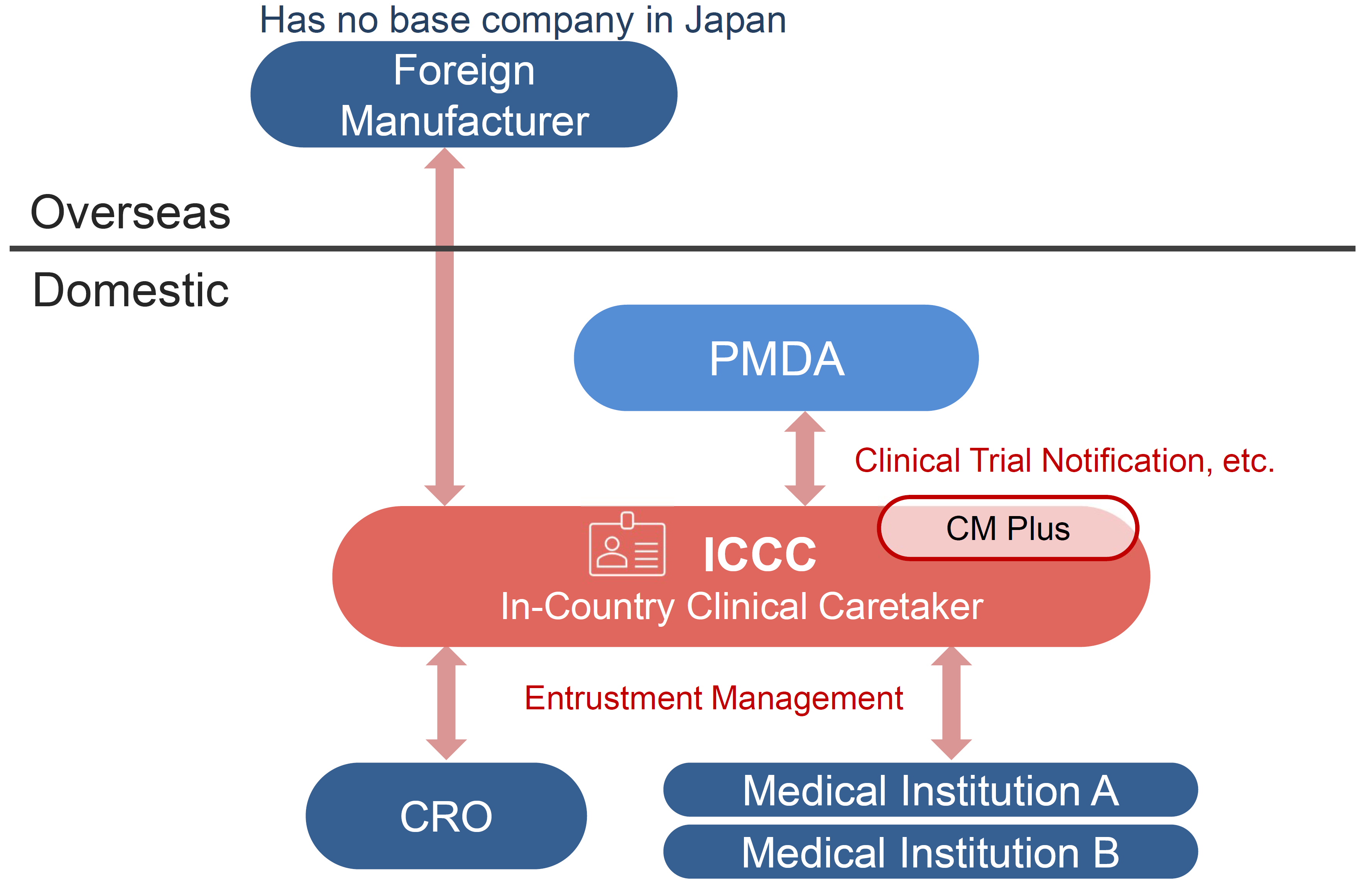
When a company without a legal entity in Japan conducts a clinical trial in Japan, it is necessary to prevent the occurrence or spread of health hazards caused by the investigational drug. It is required to appoint a person addressed in Japan as a person who can request a clinical trial on behalf of the person.
As a clinical development partner, CM Plus will play the sponsor role in Japan (ICCC: Article 15 in GCP Ministerial Ordinance) on behalf of a pharmaceutical company without a business base in Japan.
Do you have following problems?
For Overseas Companies

Even if We have no business base company in Japan, can We obtain Manufacturing/ marketing approval of pharmaceutical products without out-licensing the product?

CM Plus has obtained business licenses for the first-class and second-class marketing licenses for pharmaceuticals. Appointing CM Plus as the Designated Marketing Authorization Holder (D-MAH) makes it possible to apply for foreign special approval. You can use it to apply for Marketing approval in Japan.

Is it possible to conduct a clinical trial in Japan even if there is no company based in Japan?

By appointing CM Plus as the In-Country Clinical Caretaker (ICCC), clinical trials can be conducted in Japan as needed. Also, we have staff with abundant experience in development and marketing, and CMP can propose a clinical trial implementation system (CRO, medical institution, testing institution) in Japan.
Thank you.
We're looking forward to a successful partnership.